
What is the average atomic mass of M Consider a gaseous binary compound with a molar mass of 62.09. To find its molecular weight, you would add 12.01 + 2(16.00) = 44.01 g/mol. The products of the reaction are carbon dioxide (C). In the case of carbon dioxide, each molecule is made up of one carbon atom and two oxygen atoms, hence, the formula of carbon dioxide is a combination of 'C' and 2 'O's', or 'CO2'. The difference is that carbon dioxide has two oxygen atoms instead of just one. The molecular mass of CO is 12.01 + 16.00 or 28.01 g/mol.Īnother common compound with the same elements is CO 2 (carbon dioxide).
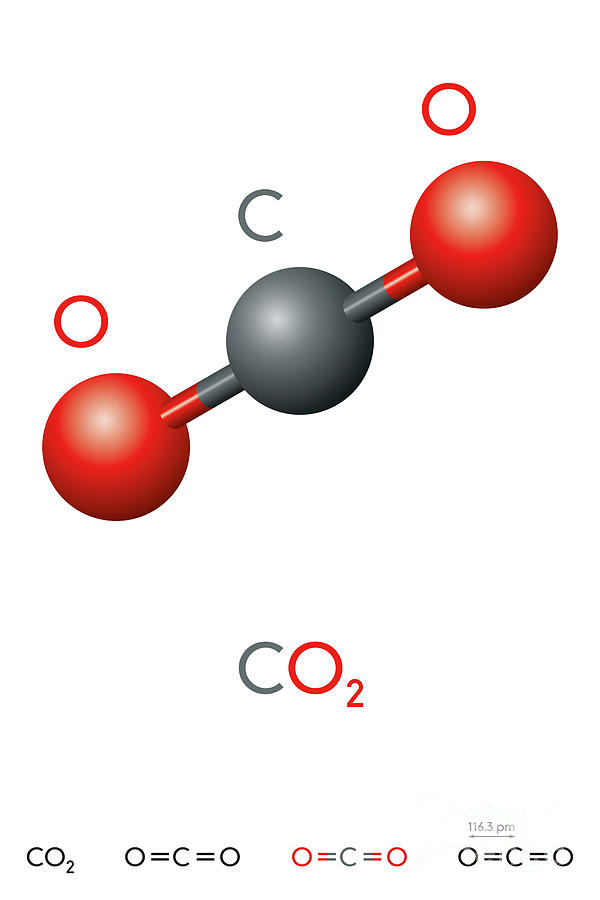
You asked about CO, which is carbon monoxide, a molecular compound that has one atom of carbon (C) and one atom of oxygen (O).įrom the table, you can see that C (element number 6) has a mass of 12.01 grams per mole, and O (element number 8) has a mass of 16.00 g/mole. We use the term "mole" so that we don't have count all of those individual atoms or molecules (it's sort of like using "miles" to specify long distances, instead of using "inches" to describe the same distance). A mole is a very large quantity of individual items (6.02 x 10 23). Equation 1 is a balanced equation one mole of methane reacts with two moles of dioxygen to produce one mole of carbon dioxide and two moles of water. Carbon dioxide,liquid - Physico-chemical Properties Molecular Formula, CO2 Molar Mass, 44.01 Density, 1.854g/cm Melting Point, 78.5 C(lit.) Boling Point. To find the molecular weight of a compound (a combination of elements), you just add the weights of the elements of the elemnts of which it consists. The mass (or weight) of an element is shown in most periodic tables, beneath the symbol. It is a chart that displays important information about all of the elements that make up our universe, including a few at the end that scientists have been able to create from other elements. You should have a periodic table of the elements in your textbook, or perhaps on the wall of your classroom.


 0 kommentar(er)
0 kommentar(er)
